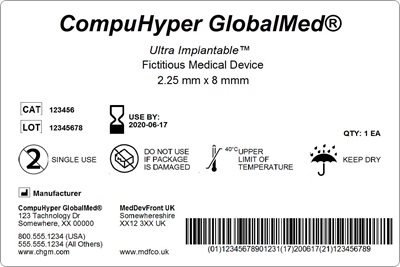
40 which identifier is used to mark the label of a controlled substance
Chapter 7 | Defense Security Cooperation Agency Prior to export, the Implementing Agency obtains import authorizations from the purchaser (21 U.S.C. 953(a)(3) and (e)(1)) and submits the documentation to the Department of Justice’s Drug Enforcement Administration and requests export permits for the controlled substance. Once approved, the export permit number, the expiration date, and the port of export should be … EUR-Lex - 32008R1272 - EN - EUR-Lex - Europa Testing that is carried out for the sole purpose of this Regulation should be carried out on the substance or mixture in the form(s) or physical state(s) in which the substance or mixture is placed on the market and in which it can reasonably be expected to be used. It should, however, be possible to use, for the purpose of this Regulation, the results of tests that are carried out to …
Statutes & Constitution :View Statutes : Online Sunshine (1) A law enforcement agency shall prepare a report identifying each prescribed controlled substance listed in Schedule II, Schedule III, or Schedule IV of s. 893.03 which is found on or near the deceased or among the deceased’s possessions. The report must identify the person who prescribed the controlled substance, if known or ascertainable.
Which identifier is used to mark the label of a controlled substance
EUR-Lex - 32017R0745 - EN - EUR-Lex - Europa Any device which, when placed on the market or put into service, incorporates, as an integral part, a substance which, if used separately, would be considered to be a medicinal product as defined in point 2 of Article 1 of Directive 2001/83/EC, including a medicinal product derived from human blood or human plasma as defined in point 10 of Article 1 of that Directive, and that has an … Guidance on Drug Establishment Licences (GUI-0002) 01.04.2020 · Controlled drug A substance included in Schedule I, II, III, IV or V of the Controlled Drugs and Substances Act. Distributor. A person, including an association or partnership, who under their own name, or under a trade, design or word mark, trade name or other name, word or mark controlled by them, sells a food or drug. Divisions 1A and 2 to 4 of the FDR apply to the … NYS Pharmacy:Laws, Rules & Regulations:Article 137 - New York … 04.03.2022 · A drug dispensed on a written or oral prescription of a physician, dentist, podiatrist or veterinarian (except a controlled substance), shall be exempt from the requirements of this section if such drug bears a label containing the name and place of business of the dispenser, the serial number and date of the prescription, directions for use as may be stated in the …
Which identifier is used to mark the label of a controlled substance. Maritime Occupational Health and Safety Regulations 4 (1) If an employer is required by section 125 or 125.1 of the Act to the employer must keep and maintain the record and make it readily available for examination by the Head of Compliance and Enforcement, the policy committee or, if there is no policy committee, the work place committee or the health and safety representative for the vessel to which it applies. Investigational Medicinal Product (IMPD) Guideline - Pharma … 31.10.2020 · Ship in a controlled manner as per the shipping order and data loggers shall be used during transit. In case a transfer of Investigational Medicinal Product (IMPD) is required from one site to another, it shall be done as per the relevant SOPs of the respective departments of FDD responsible for conducting the trial. Complaint handling, recalls, and returns shall be done as … CFR - Code of Federal Regulations Title 21 - Food and Drug ... Mar 29, 2022 · (i) For each controlled substance in bulk form to be used in (or capable of use in) the manufacture of the same or other controlled or non-controlled substances in finished form, the inventory shall include: (A) The name of the substance and (B) The total quantity of the substance to the nearest metric unit weight consistent with unit size. Dy-Mark Spray & Mark Water Based All Colours Dy-Mark Dy-Mark Chemwatch Hazard Alert Code: 4 Dy-Mark Spray & Mark Water Based All Colours Chemwatch: 04-0171 Version No: 14.1.1.1 Safety Data Sheet according to WHS and ADG requirements Issue Date: 31/08/2020 Print Date: 05/11/2020 S.GHS.AUS.EN SECTION 1 Identification of the substance / mixture and of the company / undertaking Product Identifier …
DCMI: DCMI Metadata Terms - Dublin Core 20.01.2020 · The Uniform Resource Identifier used to uniquely identify a term. Definition: A statement that represents the concept and essential nature of the term. Type of Term: The type of term: property, class, datatype, or vocabulary encoding scheme. Where applicable, the following attributes provide additional information about a term: Comment: Additional information about … eCFR :: 21 CFR Part 1304 -- Records and Reports of Registrants 06.04.2009 · On the effective date of a rule by the Administrator pursuant to §§ 1308.45, 1308.46, or 1308.47 of this chapter adding a substance to any schedule of controlled substances, which substance was, immediately prior to that date, not listed on any such schedule, every registrant required to keep records who possesses that substance shall take an inventory of all stocks of … NYS Pharmacy:Laws, Rules & Regulations:Article 137 - New York … 04.03.2022 · A drug dispensed on a written or oral prescription of a physician, dentist, podiatrist or veterinarian (except a controlled substance), shall be exempt from the requirements of this section if such drug bears a label containing the name and place of business of the dispenser, the serial number and date of the prescription, directions for use as may be stated in the … Guidance on Drug Establishment Licences (GUI-0002) 01.04.2020 · Controlled drug A substance included in Schedule I, II, III, IV or V of the Controlled Drugs and Substances Act. Distributor. A person, including an association or partnership, who under their own name, or under a trade, design or word mark, trade name or other name, word or mark controlled by them, sells a food or drug. Divisions 1A and 2 to 4 of the FDR apply to the …
EUR-Lex - 32017R0745 - EN - EUR-Lex - Europa Any device which, when placed on the market or put into service, incorporates, as an integral part, a substance which, if used separately, would be considered to be a medicinal product as defined in point 2 of Article 1 of Directive 2001/83/EC, including a medicinal product derived from human blood or human plasma as defined in point 10 of Article 1 of that Directive, and that has an …















/200415150-001-56a6fd333df78cf772914d1a.jpg)
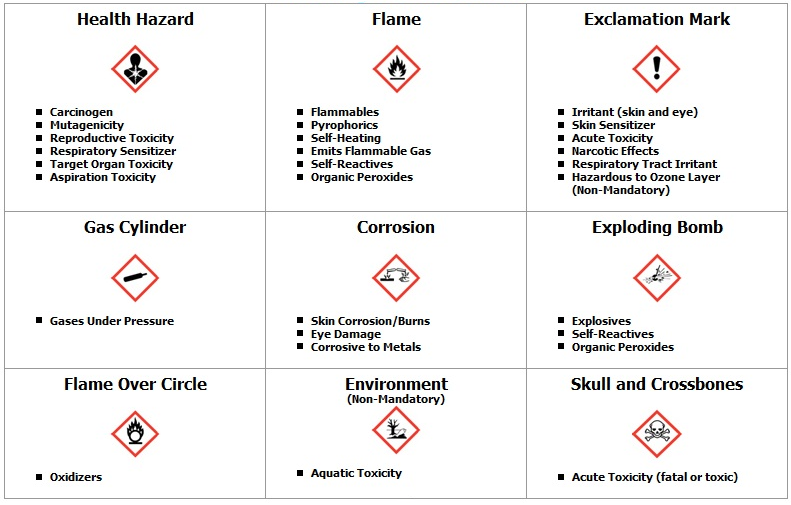




Post a Comment for "40 which identifier is used to mark the label of a controlled substance"