40 exempt human specimen meaning
grants.nih.gov › policy › humansubjectsHuman Subjects Research - Home page | grants.nih.gov Aug 02, 2021 · Learn more about research that meets the definition human subjects research, Federal regulation requirements, and whether your project may be considered exempt. Also, learn about NIH-specific considerations and become more familiar with NIH policies, and other regulations as it relates to human subjects research protections. › quarantine › pdfCENTERS FOR DISEASE CONTROL AND PREVENTION DEPARTMENT OF ... Jan 02, 2021 · department of health and human services . order under section 361 . of the public health service act (42 u.s.c. § 264) and 42 code of federal regulations §§ 71.20 & 71.31(b) requirement for negative pre-departure covid-19 test result . or documentation of recovery from covid-19 . for all airline or other aircraft passengers arriving
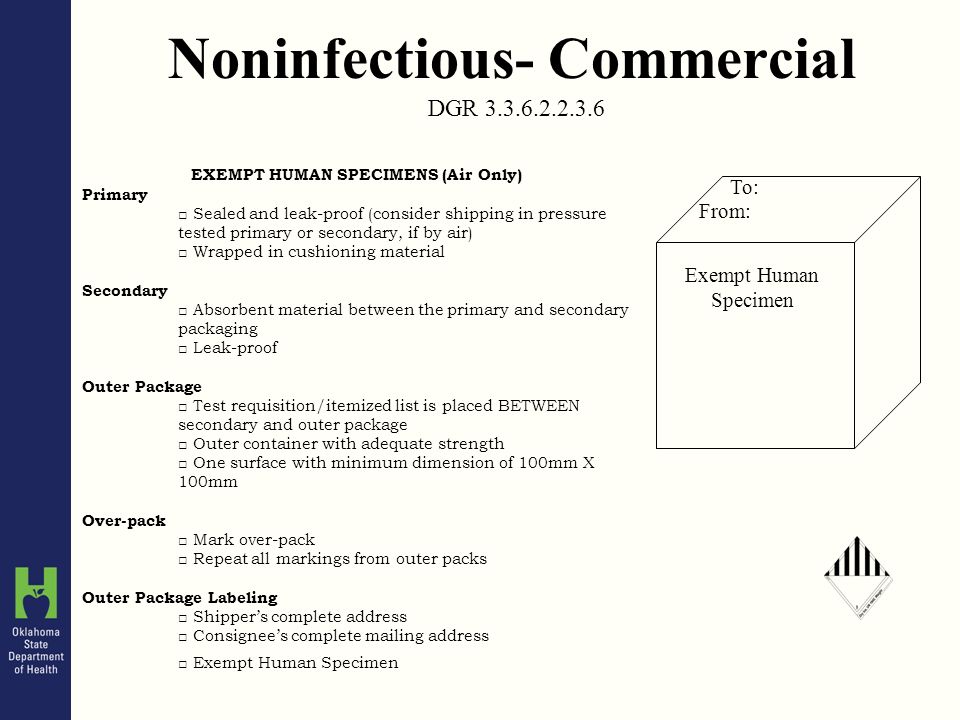
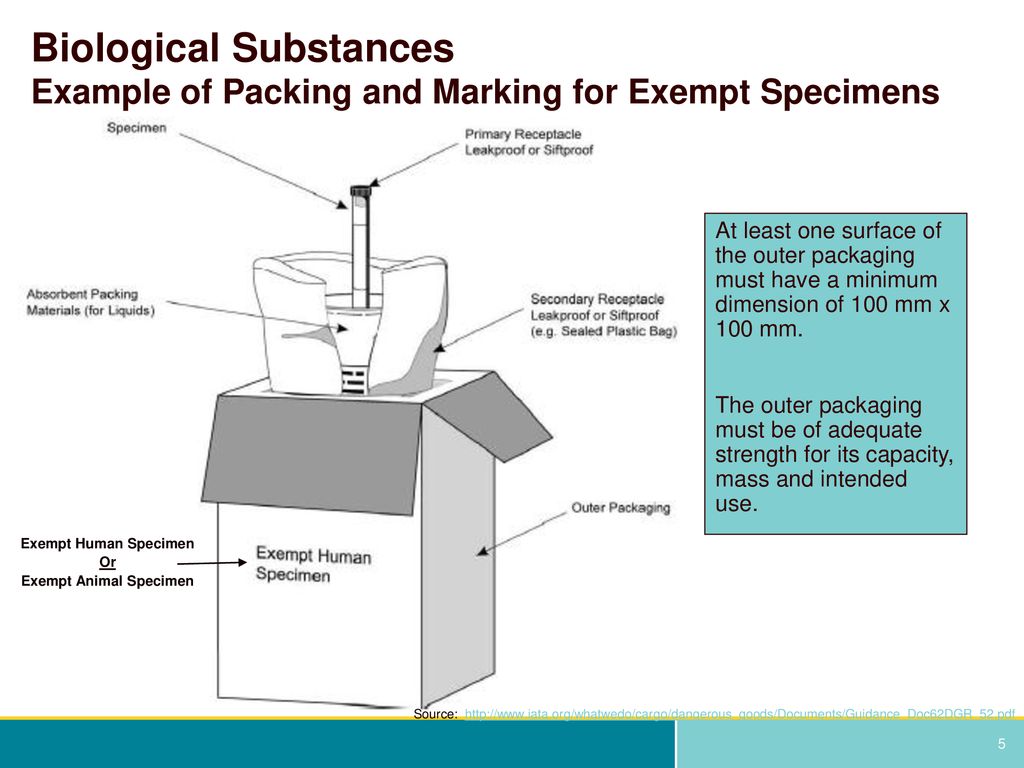
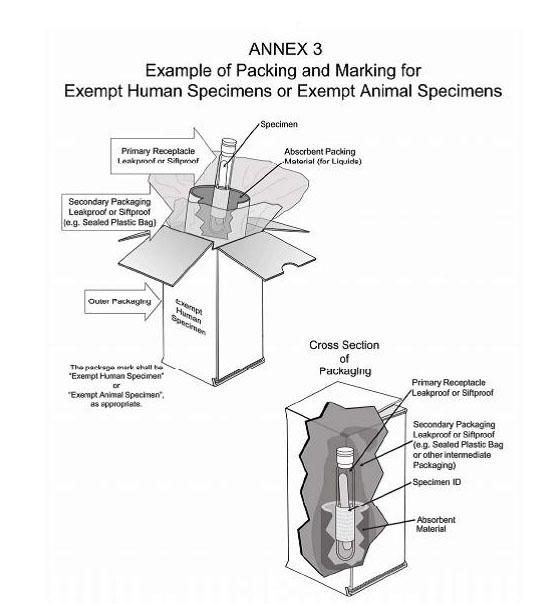
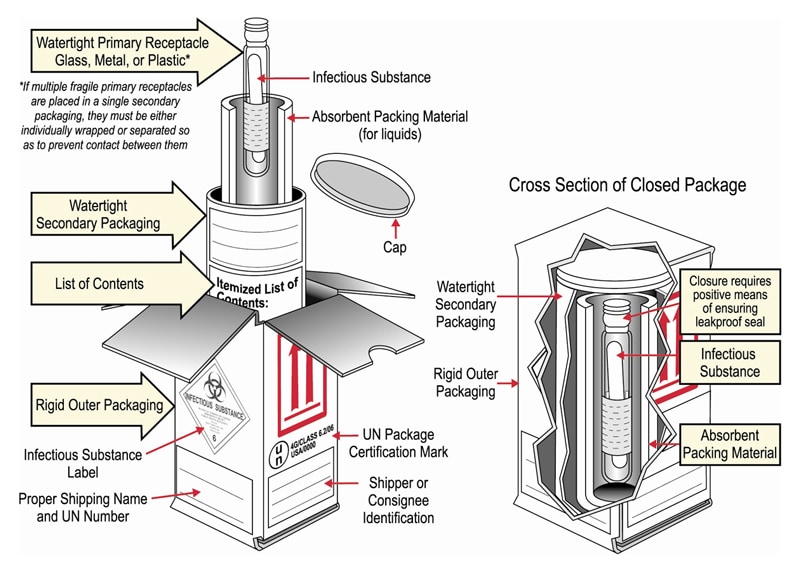
Packaging and transport requirements for patient samples - GOV.UK the mark (s) can be preprinted onto the outer packaging. (5) At least one surface of the outer packaging shall have a minimum dimension of 100 mm x 100 mm. (6) The completed package shall be ...

Exempt human specimen meaning
exempt human specimen - Greek translation - Linguee Article 135(1)(i) of Council Directive 2006/112/EC of 28 November 2006 on the common system of value added tax must be interpreted as meaning that the exercise of the discretionary power of the Member States to fix conditions and limitations on the exemption from value added tax provided for by that provision allows those States to exempt from that tax only certain forms of gambling. exempt human specimen - Traduzione in italiano - Dizionario Linguee Moltissimi esempi di frasi con "exempt human specimen" - Dizionario italiano-inglese e motore di ricerca per milioni di traduzioni in ... uniform basis of assessment must be interpreted as meaning that the fact that an insurance broker or agent does not have a direct relationship with the parties to the insurance or reinsurance contract in ... What is "Exempt" Human Subject Research, And What Does It Mean? (2019 ... what is "exempt" human subjects research, and what does it mean? Briefly, research is termed "Exempt" when it constitutes research with human subjects, but ALSO meets the requirements of a defined low-risk category that is exempt from SOME (but not all) of the requirements governing human subjects research.
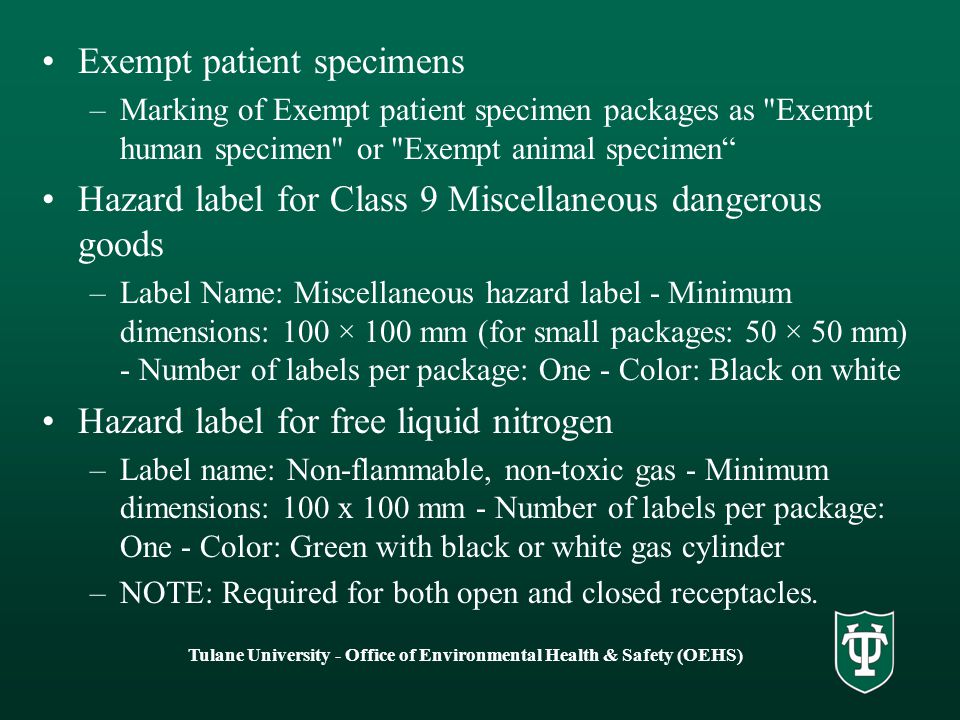
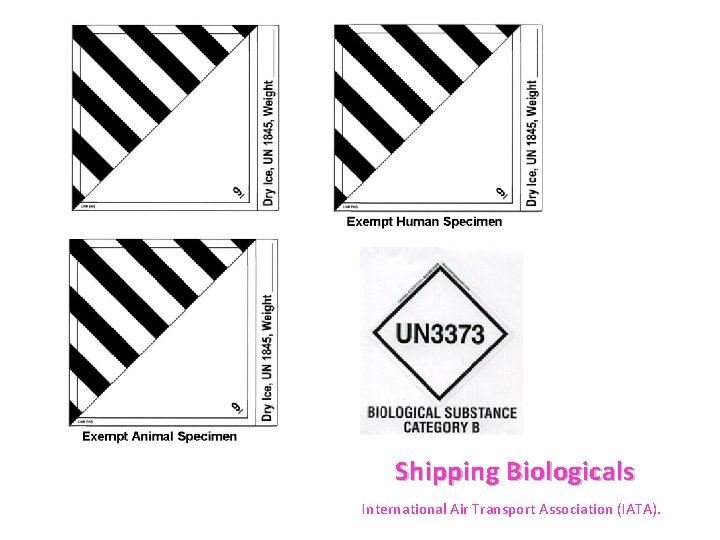
Exempt human specimen meaning. PDF 3 For the purposes of these Regulations - IATA the words "Exempt human specimen" or "Exempt 3.6.2.2.3.3 Substances in a form that any present patho- animal specimen," as appropriate; gens have been neutralized or inactivated such that they › ohrp › regulations-and-policyThe Belmont Report | HHS.gov The Belmont Report was written by the National Commission for the Protection of Human Subjects of Biomedical and Behavioral Research. The Commission, created as a result of the National Research Act of 1974, was charged with identifying the basic ethical principles that should underlie the conduct of biomedical and behavioral research involving human subjects and developing guidelines to ... Exempt Animal or Human Specimens | Environment, Health and Safety Exempt Animal or Human Specimens. Patient specimens (containing no other hazardous materials) for which there is minimal likelihood that pathogens are present are not subject to other shipping regulations except: The specimen must be packed in a packaging which will prevent any leakage and which is marked with the words: "Exempt Human Specimen", or. Patient specimen Definition | Law Insider Exempt human or animal specimens are not subject to regulation as hazardous materials but must be packaged according to 346.326.e. Patient specimen means material that is collected directly from humans or animals and transported for purposes such as diagnosis and research.
exempt human specimen - Traduction française - Linguee 2.6.3.2.3.6 Human or animal specimens for which there is minimal likelihood that pathogens are present are not subject to these Regulations if the specimen is transported in a packaging which will prevent any leakage and which exempt human specimen - French translation - Linguee is marked with the wo rds "Exempt human specimen" or "Exempt anim al specimen", as appropriate. daccess-ods.un.org. daccess-ods.un.org. 2.6.3.2.3.6 Les échantillons humains ou animaux qui présentent un risque minimal de contenir des agents pathogènes ne sont pas soumis au présent Règlement s'ils sont transportés dans un emballage conçu. PDF Step 3: Packing Category A and B and Exempt Human and Exempt Animal ... Step 3: Packing Category A and B and Exempt Human and Exempt Animal Specimens Job Aid . Use the pages below as a reference for packing Category A, B, and Exempt Specimens. Category A Substance Packaging . NOTE: The packaging is the same for both types (UN 2814 and UN2900) of Category A packaging, only the UN mark and Proper Shipping Names change. Shipping Infectious Substances - Transport Canada Human or animal specimens are exempted from certain parts of the TDG Regulations if you have no reason to believe that the specimen contains an infectious substance. ... The term "reason to believe" means that there is sufficient belief to suggest that the specimens contain infectious substances included in Category A or B.
exempt human specimen - Ελληνική μετάφραση - Linguee Πολλές ενδεικτικές μεταφρασμένες προτάσεις που περιέχουν «exempt human specimen» - Ελληνο-Αγγλικό λεξικό και μηχανή αναζήτησης για ελληνικές μεταφράσεις. Coded Private Information or Specimens Use in Research, Guidance (2008 ... Having determined under the second question above that a research activity involves human subjects because the investigators are obtaining identifiable private information or specimens, assessment under the exemption at 45 CFR 46.101(b)(4) focuses, in part, on: (1) whether the data or specimens are existing at the time the research is proposed to an institutional official or IRB for a determination of whether the research is exempt, and (2) how the data or information is recorded by the ... Exempt patient specimens - un3373.it exempt human specimen or exempt animal specimen They are collected directly from humans or animals and there is minimal likelihood that pathogens are present. An element of professional judgment is required to determine if a substance is exempt under this paragraph. Definition of Human Subjects Research | grants.nih.gov According to 45 CFR 46, a human subject is "a living individual about whom an investigator (whether professional or student) conducting research: Obtains information or biospecimens through intervention or interaction with the individual, and uses, studies, or analyzes the information or biospecimens; or
Import Permit Program - Frequently Asked Questions | CDC According to the import permit regulations, an import permit is not required for a diagnostic specimen (with the exception of bat or nonhuman primate specimens) not known by the importer to contain, or suspected by the importer of containing, an infectious biological agent and accompanied by a certification statement confirming that the material is not known to contain or suspected of ...
› statutes › indexStatutes & Constitution :View Statutes : Online Sunshine (b) Except as provided in s. 775.16, a person may be denied a license, permit, or certification to pursue, practice, or engage in an occupation, trade, vocation, profession, or business by reason of the prior conviction for a crime if the crime was a felony or first-degree misdemeanor that is directly related to the standards determined by the regulatory authority to be necessary and ...
USPS Packaging Instruction 6H | Postal Explorer Typically, exempt human specimens are specimens for which there is a low probability that the sample is infectious, such as specimens for drug or alcohol testing; cholesterol testing; blood glucose level testing; prostate-specific antigens (PSA) testing; testing to monitor heart, kidney, or liver function; pregnancy testing; and testing for diagnosis of noninfectious diseases such as cancer biopsies.
How to Ship Clinical Samples | FedEx This document outlines the guidelines for shipping with FedEx Express. For the purposes of this guide, clinical samples are generally defined as non-infectious human or animal materials including, but not limited to, excreta, secreta, tissue and tissue fluids, blood and FDA-approved pharmaceuticals that are blood products.
PDF Packaging and Shipping Exempt Human Specimens Title: SSR-Faculty18111916510 Created Date: 11/19/2018 4:51:52 PM
› files › 1656The Project Gutenberg eBook of Apology, by Plato Oct 04, 2020 · INTRODUCTION. In what relation the “Apology” of Plato stands to the real defence of Socrates, there are no means of determining. It certainly agrees in tone and character with the description of Xenophon, who says in the “Memorabilia” that Socrates might have been acquitted “if in any moderate degree he would have conciliated the favour of the dicasts;” and who informs us in ...
› instructions › i990Instructions for Form 990 Return of Organization Exempt From ... Answer “Yes” if the organization has received a letter ruling that its obligations were issued on behalf of a state or local governmental unit; meets the conditions for issuing tax-exempt bonds as set forth in Rev. Rul. 63-20, 1963-1 C.B. 24 (see Rev. Proc. 82-26, 1982-1 C.B. 476); or is a constituted authority organized by a state or local ...
Exempt Human The Exempt Human Specimen (EHS) category has a specific packing and marking requirement. Specimen shipping packages consigned to couriers and air carriers must have the marking "Exempt Human Specimen" and must, at a minimum, meet the following package requirements: 100 mm² dimension on one side; leak-proof primary container
› research › human-subjectsHuman Subjects Certifications—IRB or IEC SOP | NIH: National ... Often residing in local institutions, IRBs and IECs independently determine whether projects are human subjects research or are exempt according to 45 CFR Part 46.101(b). For domestic sites of multi-site studies where each site will conduct the same protocol involving non-exempt human subjects research funded by NIH, the sIRB carries out the ...
What does the term "exempt" actually mean in human subjects research ... Human subjects research that is classified as "exempt" means that the research qualifies as no risk or minimal risk to subjects and is exempt from most of the requirements of the Federal Policy for the Protection of Human Subjects, but is still considered research requiring an IRB review for an exemption determination.
PDF Exempt Human Specimen / Exempt Animal Specimen Reference Guide (IATA 3 ... Exempt Human Specimen / Exempt Animal Specimen Reference Guide (IATA 3.6.2.2.3.6) 1. Package is marked with the words "Exempt human specimen" or "Exempt animal specimen", as appropriate. (this would be in lieu of a UN3373 label). 2. The packaging must consist of three components: a. a leak-proof primary receptacle(s);
Reproductive human cloning Definition - Law Insider definition. Reproductive human cloning means human cloning intended to result in the gestation or birth of a child who is genetically identical to another conceptus, embryo, fetus, or human being, living or dead.
What is "Exempt" Human Subject Research, And What Does It Mean? (2019 ... what is "exempt" human subjects research, and what does it mean? Briefly, research is termed "Exempt" when it constitutes research with human subjects, but ALSO meets the requirements of a defined low-risk category that is exempt from SOME (but not all) of the requirements governing human subjects research.
exempt human specimen - Traduzione in italiano - Dizionario Linguee Moltissimi esempi di frasi con "exempt human specimen" - Dizionario italiano-inglese e motore di ricerca per milioni di traduzioni in ... uniform basis of assessment must be interpreted as meaning that the fact that an insurance broker or agent does not have a direct relationship with the parties to the insurance or reinsurance contract in ...
exempt human specimen - Greek translation - Linguee Article 135(1)(i) of Council Directive 2006/112/EC of 28 November 2006 on the common system of value added tax must be interpreted as meaning that the exercise of the discretionary power of the Member States to fix conditions and limitations on the exemption from value added tax provided for by that provision allows those States to exempt from that tax only certain forms of gambling.
















Post a Comment for "40 exempt human specimen meaning"