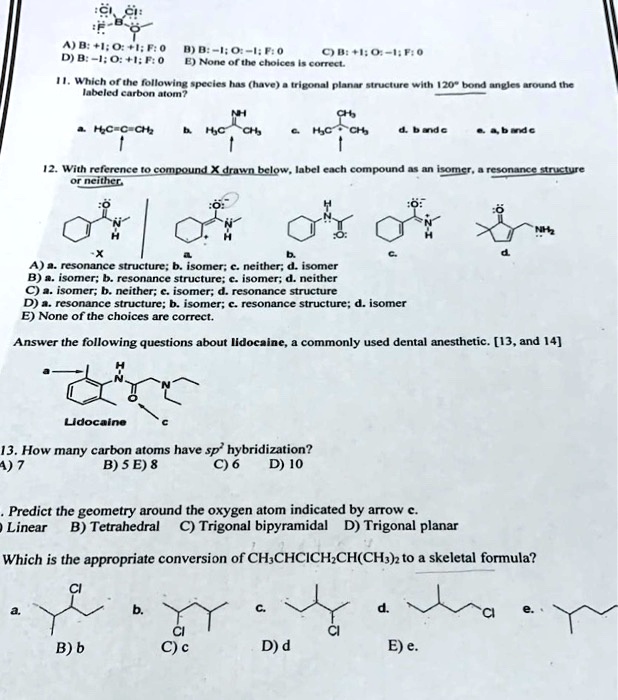
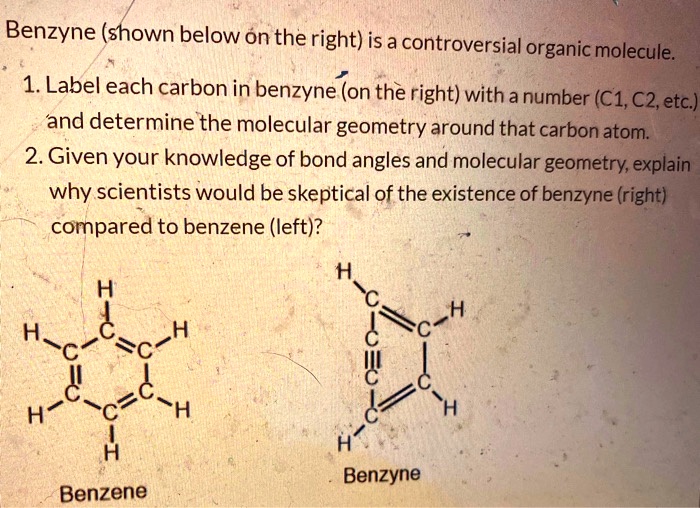
38 label each carbon atom with the appropriate geometry.
OChem Spring 2017 Exam 1 Flashcards - Quizlet Label each carbon atom wiht the appropriate geometry (cover right side) Sapling Hw Ch 1.21 Predict the molecular shape of methane, the carbonate ion, carbon dioxide, and the sulfite ion (cover left side of screen) How to Identify Chiral Carbons | Identify Chiral Carbons ... - Pediaa.Com Step 1. First, determine whether the groups attached to the carbon atom are different from each other. If they are different, we can guess that it as a chiral carbon. In the above image, the molecule has a hydrogen atom and a methyl group attached to the same carbon atom. But other two groups have formed a ring.
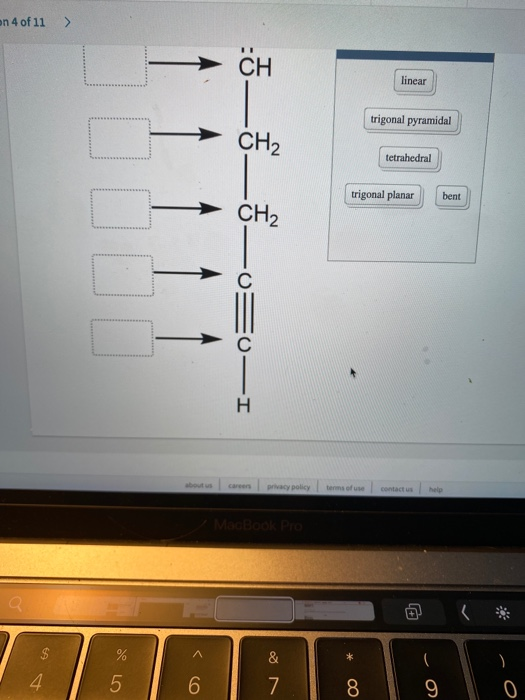
Answered: Label each carbon atom with the… | bartleby Solution for Label each carbon atom with the appropriate geometry. CH2 Answer Bank CH tetrahedral linear trigonal planar bent CH2 trigonal pyramidal CH2 C C H.

Label each carbon atom with the appropriate geometry.
Label each carbon atom with the appropriate geometry. Answer to Label each carbon atom with the appropriate geometry. trigonal planar CH2 trigonal planar CH trigonal planar linear tetrahedral ČH2 tetrahedral bent tetrahedra | SolutionInn HCN Lewis Structure, Molecular Geometry, Shape, and Polarity HCN Molecular Geometry. The molecular Geometry of any given molecule helps understand its three-dimensional structure and the arrangement of atoms in a molecule, and its shape. Hydrogen Cyanide has geometry like AX2 molecule, where A is the central atom and X is the number of atoms bonded with the central atom. As Carbon is bonded to two atoms ... [ANSWERED] Label The Molecular Shape Around Each Of The Central Atoms ... So, geometry around nitrogen on position (1) is trigonal pyramidal. Hybridization of Carbon at position (2) is s{p^3} s p 3 . So, geometry aroundCarbon at position (2) is tetrahedral. Hybridization of Carbon at position (3) is s{p^2} s p 2 and has trigonal planer geometry. Hybridization of Oxygen at position (4) is s{p^3} s p 3 and has bent ...
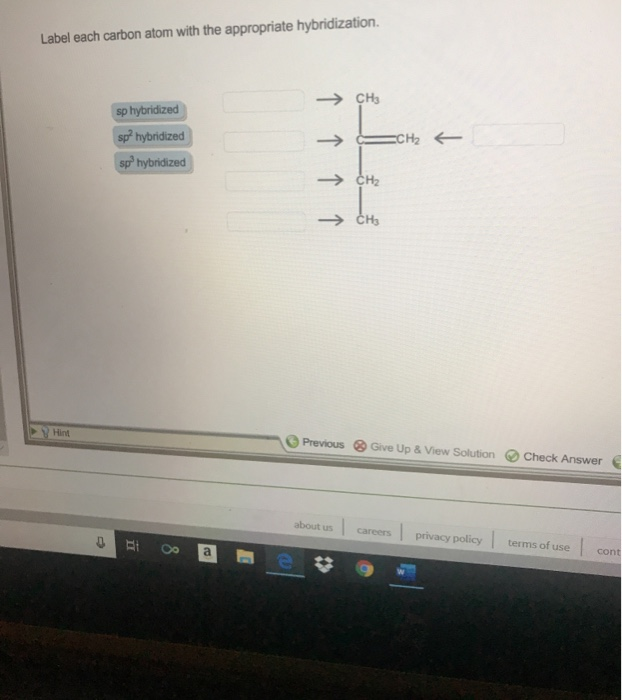
Label each carbon atom with the appropriate geometry.. Carbon dioxide (CO2) lewis dot structure, molecular geometry, bond ... The electron geometry of CO2 is also linear. In the CO2 lewis structure, there is a total of 4 lone pairs present. Two lone pairs on each oxygen atom. The bond angle of CO2 is 180º. Since it is linear in shape with an arrangement like that O=C=O. Two types of hybridization in CO2 - Sp, and Sp2. Molecular Geometry - Chem1 Tetrahedrally-coordinated carbon chains. Carbon atoms are well known for their tendency to link together to form the millions of organic molecules that are known. We can work out the simpler hydrocarbon chains by looking at each central atom separately. Thus the hydrocarbon ethane is essentially two CH 3 tetrahedra joined end-to-end. (Get Answer) - Label each carbon atom with the appropriate geometry ... Label Each Carbon Atom With The Appropriate Geometry. Trigonal Planar > GHz Trigonal Planar > CH Trigonal Planar Linear Tetrahedral > CH2 Tetrahedral 111111 Tetrahedral Bent CH2 Trigonal Pyramidal Linear Bent > CH GHz trigonal planar > CH trigonal... label each carbon atom with the appropriate hybridization 🚀To book a personalized 1-on-1 tutoring session:👉Janine The Tutorhttps://janinethetutor.com🚀More proven OneClass Services you might be interested in:👉One...
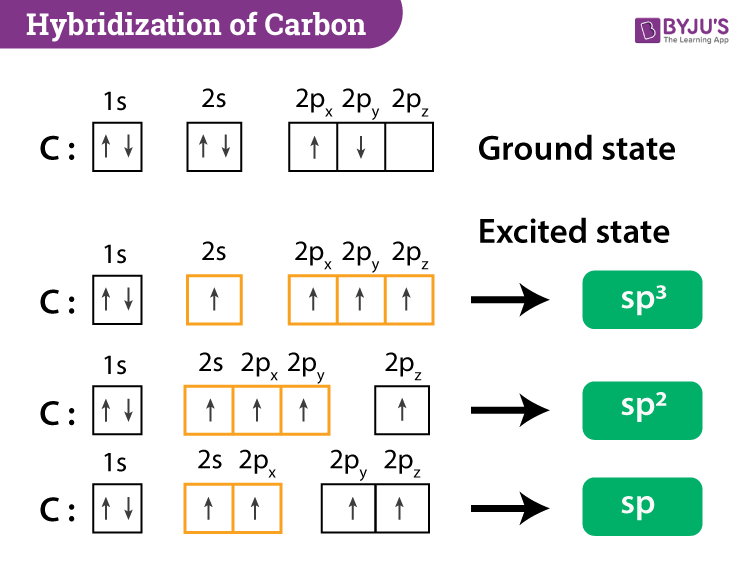
Chapter 9 Homework Flashcards - Questions and Answers - Quizlet There are 6 C atoms in the molecule. Starting on the left, the hybridizations are: sp2, sp2, sp3, sp, sp, sp3. All single bonds are bonds. Double and triple bonds each contain 1 bond. This molecule has 8 C-H bonds and 5 C-C bonds, for a total of 13 bonds. Double bonds have 1 bond and triple bonds have 2 bonds. This molecule has a total of 3 bonds. Finding the hybridization of atoms in organic molecules ... - Khan Academy And if it's SP two hybridized, we know the geometry around that carbon must be trigonal, planar, with bond angles approximately 120 degrees. This carbon over here, also has a double-bond to it, so it's also SP two hybridized, with trigonal planar geometry. Label each carbon atom with the appropriate geometry ... - Course Hero Fundamentals The geometry of sp3 hybridized carbon atom is tetrahedral. The geometry of sp2 the hybridized carbon atom is trigonal planar. The geometry of sp hybridized carbon atom is linear. Step-by-step explanation Step 2 The hybridizations of each carbon in the molecule are as follows Step 3 The geometry of each carbon atoms is as follows: Hybridization of Carbon - Molecular Geometry and Bond Angles 1. sp Hybridization Carbon can have an sp hybridization when it is bound to two other atoms with the help of two double bonds or one single and one triple bond. When the hybridization occurs the molecules have a linear arrangement of the atoms with a bond angle of 180°. Example: Hybridization of CO 2. 2. sp2 Hybridization
Label each carbon atom with the appropriate geometry. - Transtutors Label each carbon atom with the appropriate geometry. Trigonal pyrimidal Trigonal planar Tetrahedral Linear Bent CH2 (double bond) CH (single bond) CH2 (single bond) CH2 (single bond) C (triple bond) CH The Structure of an Atom Explained With a Labeled Diagram The nucleus is very small compared to the size of atom and the entire mass of an atom is centered in the nucleus. This conclusion helped him propose 'Rutherford's Atomic Model'. According to his atom diagram, the atom has a small, positively charged nucleus in center. This nucleus carries the entire mass of the atom. ⚗️Label each carbon atom with the appropriate geometry. Bin 1 points to ... Label each carbon atom with the appropriate geometry. Bin 1 points to a carbon bonded to a double bonded carbon and single bonded to two hydrogens. Bin 2 points to a carbon double bonded to a carbon and single bonded to a carbon and one hydrogen. Bin 3 is a carbon single bonded to two carbons and single bonded to two hydrogens. Label each carbon atom with the appropriate geometry. - OneClass 5 Nov 2019. Label each carbon atom with the appropriate geometry. Trigonal pyrimidal. Trigonal planar. Tetrahedral. Linear. Bent. CH2 (double bond) CH (single bond) CH2 (single bond) CH2 (singlebond) C (triple bond) CH. Show full question.
Solved Label each carbon atom with the appropriate geometry. - Chegg See the answer. See the answer See the answer done loading. Label each carbon atom with the appropriate geometry. Show transcribed image text.
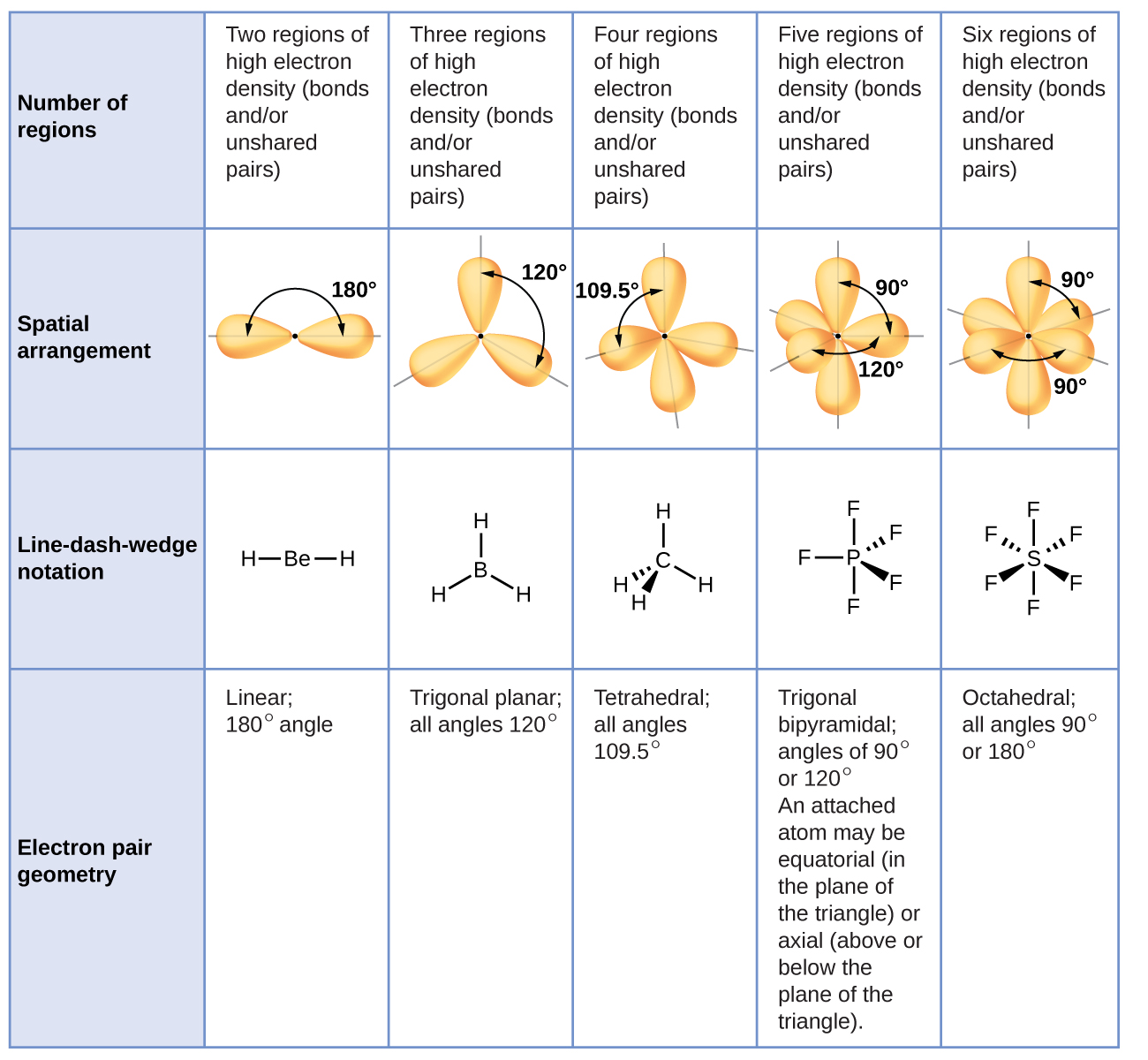
Molecular Geometry - Oklahoma State University-Stillwater The shape we see is the only possible shape for a central carbon atom with four bonds. This geometry is a direct result of the repulsion experienced by the four groups of bonding electrons. The shape of this molecule is a result of the electrons in the four bonds positioning themselves so as to minimize the repulsive effects.
E-Z notation for geometric isomerism - chemguide The atoms attached directly to the carbon of the CH 2 group are C H H. In the second list, the C is written first because it has the highest atomic number. Now compare the two lists atom by atom. The first atom in each list is an H in the CH 3 group and a C in the CH 3 CH 2 group. The carbon has the higher priority because it has the higher ...
7.6 Molecular Structure and Polarity - Chemistry Predict the local geometry for the nitrogen atom, the two carbon atoms, and the oxygen atom with a hydrogen atom attached: Solution. Consider each central atom independently. The electron-pair geometries: nitrogen--four regions of electron density; tetrahedral; carbon (CH 2)--four regions of electron density; tetrahedral
Quiz1 88%.docx - Question 1 Label each carbon atom with the appropriate ... Question 1 Label each carbon atom with the appropriate hybridization. Question 2 Determine the formal charge on each atom in the. Study Resources. Main Menu; by School; by Literature Title; ... Molecular Geometry and Bonding Theories; Albany State University • CHEM 1212K. Chem Chpt 9 Lecture Notes .docx. notes. 7. Chapter 8 Notes. Albany ...
What is the geometry around each of the three central atoms in the CH ... Carbon 1 This atom has four atoms directly attached and no lone pairs. Its electron geometry and its molecular geometry are both tetrahedral as in methane. Carbon 2 This atom has three atoms directly attached and no lone pairs. Its electron geometry and its molecular geometry are both trigonal planar. Oxygen 3
[ANSWERED] Label The Molecular Shape Around Each Of The Central Atoms ... So, geometry around nitrogen on position (1) is trigonal pyramidal. Hybridization of Carbon at position (2) is s{p^3} s p 3 . So, geometry aroundCarbon at position (2) is tetrahedral. Hybridization of Carbon at position (3) is s{p^2} s p 2 and has trigonal planer geometry. Hybridization of Oxygen at position (4) is s{p^3} s p 3 and has bent ...
HCN Lewis Structure, Molecular Geometry, Shape, and Polarity HCN Molecular Geometry. The molecular Geometry of any given molecule helps understand its three-dimensional structure and the arrangement of atoms in a molecule, and its shape. Hydrogen Cyanide has geometry like AX2 molecule, where A is the central atom and X is the number of atoms bonded with the central atom. As Carbon is bonded to two atoms ...
Label each carbon atom with the appropriate geometry. Answer to Label each carbon atom with the appropriate geometry. trigonal planar CH2 trigonal planar CH trigonal planar linear tetrahedral ČH2 tetrahedral bent tetrahedra | SolutionInn























Post a Comment for "38 label each carbon atom with the appropriate geometry."